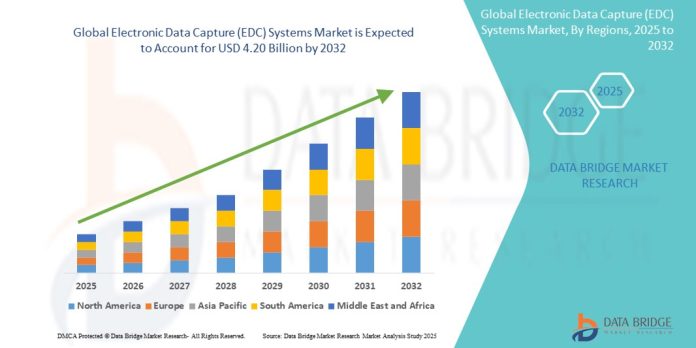
The global Electronic Data Capture (EDC) Systems Market is experiencing robust growth as the healthcare and life sciences sectors increasingly embrace digital transformation. EDC systems are software solutions designed to collect clinical trial data electronically, replacing the traditional paper-based methods. These systems streamline clinical research processes, improve data accuracy, reduce time to market, and comply with regulatory requirements. With the growing demand for faster and more cost-efficient clinical trials, EDC systems have become a cornerstone of modern research operations.
This article explores the key trends, growth drivers, challenges, opportunities, and future outlook of the Electronic Data Capture (EDC) Systems Market.
Understanding Electronic Data Capture (EDC) Systems
Electronic Data Capture (EDC) systems are digital platforms that collect, manage, and store patient and trial data during clinical research. They enable real-time data access, minimize manual errors, and ensure compliance with regulatory standards such as Good Clinical Practice (GCP) and the FDA’s 21 CFR Part 11.
These systems are widely used in pharmaceutical companies, biotechnology firms, clinical research organizations (CROs), and academic research institutions. Their ability to handle large, complex datasets makes them essential for modern drug development and medical device trials.
Market Growth Drivers
Several factors are fueling the growth of the Electronic Data Capture (EDC) Systems Market:
1. Rising Number of Clinical Trials
The global pharmaceutical and biotechnology sectors are investing heavily in research and development (R&D). According to clinical trial registries, the number of ongoing clinical trials worldwide has grown substantially in the past decade. EDC systems are increasingly preferred to handle the vast amount of data generated from these studies.
2. Increasing Demand for Cost Efficiency
Conducting a clinical trial is an expensive and time-consuming process. By digitizing data collection and reducing human error, EDC systems help organizations cut costs, accelerate trial completion, and improve overall return on investment.
3. Compliance with Regulatory Standards
The growing complexity of clinical trials requires strict compliance with regulatory standards. EDC systems help organizations adhere to ICH-GCP guidelines, HIPAA regulations, and local data privacy laws while ensuring secure data storage and retrieval.
4. Technological Advancements
Integration of cloud computing, artificial intelligence (AI), and advanced analytics into EDC platforms is enhancing their efficiency. Features such as real-time data validation, predictive analytics, and automated monitoring are transforming how clinical data is collected and managed.
5. Growth of Decentralized and Virtual Clinical Trials
The COVID-19 pandemic accelerated the adoption of remote and decentralized clinical trials. EDC systems with mobile compatibility and remote monitoring capabilities play a crucial role in enabling virtual trials, allowing researchers to collect patient data without geographical barriers.
Key Market Segments
The Electronic Data Capture (EDC) Systems Market can be segmented based on component, deployment mode, end-user, and region.
1. By Component
- Software – The largest market share, as software solutions form the core of EDC platforms.
- Services – Includes training, maintenance, consulting, and support.
2. By Deployment Mode
- Cloud-Based EDC Systems – Gaining popularity due to scalability, flexibility, and cost-effectiveness.
- On-Premise Systems – Preferred by organizations prioritizing data security and regulatory control.
3. By End-User
- Pharmaceutical & Biotechnology Companies – Primary users due to high R&D activity.
- Clinical Research Organizations (CROs) – Adopt EDC systems to provide efficient trial management services.
- Medical Device Manufacturers – Use EDC platforms for regulatory-compliant trials.
- Academic Institutes & Research Centers – Growing adoption in collaborative and academic research.
4. By Region
- North America – Dominates the market, driven by advanced healthcare infrastructure and high R&D investments.
- Europe – Significant share due to strict regulatory standards and strong pharmaceutical presence.
- Asia-Pacific – Fastest-growing region with rising clinical research outsourcing, especially in China and India.
- Latin America & Middle East/Africa – Emerging markets with increasing focus on clinical development.
Challenges in the EDC Market
Despite strong growth potential, the market faces certain challenges:
- High Implementation Costs – Small and mid-sized companies may find it difficult to adopt advanced EDC systems.
- Data Security Concerns – Protecting sensitive patient data from breaches is a critical challenge.
- Complex Integration – Integrating EDC with other clinical trial management systems (CTMS) and electronic health records (EHRs) requires technical expertise.
- Regulatory Differences Across Regions – Compliance with multiple international regulations complicates adoption for global trials.
Future Opportunities
The Electronic Data Capture (EDC) Systems Market holds tremendous opportunities in the coming years:
- AI and Machine Learning Integration – Enabling predictive trial outcomes and automated data cleaning.
- Blockchain in Clinical Trials – Offering secure, transparent, and immutable data sharing.
- Mobile-Based EDC Solutions – Increasing adoption as patients and clinicians prefer smartphone-based platforms.
- Expansion in Emerging Markets – Growing clinical trial outsourcing in Asia-Pacific and Latin America presents huge market potential.
- Patient-Centric Trials – Adoption of wearable devices and remote patient monitoring will further boost EDC system demand.
Competitive Landscape
The market is highly competitive with the presence of established players and new entrants. Key companies include:
- Medidata Solutions (Dassault Systèmes)
- Oracle Health Sciences
- IBM Watson Health
- Veeva Systems
- Parexel International
- Clario (formerly ERT)
- Castor EDC
- eClinicalWorks
These companies focus on innovation, partnerships, and acquisitions to strengthen their market presence. Cloud-based platforms, AI-enabled analytics, and mobile accessibility are major areas of product development.
Get More Details:
Conclusion
The Electronic Data Capture (EDC) Systems Market is on a strong growth trajectory, driven by the increasing demand for efficient, compliant, and cost-effective clinical research solutions. As the pharmaceutical and biotechnology industries continue to expand, the adoption of digital data management systems will only accelerate.
With advancements in AI, blockchain, and mobile technologies, EDC systems are evolving into intelligent platforms that not only capture data but also enhance clinical decision-making. Although challenges such as cost and data security remain, the overall outlook for the market is highly positive.
The future of clinical trials is digital, and EDC systems will play a central role in shaping that future by improving research efficiency, reducing errors, and ensuring patient safety.